
Cell and Gene Therapy in Parkinson's Disease Market to Register Stunning Growth During the Study Period (2020–2034) | DelveInsight
The cell and gene therapy in Parkinson's disease market size is projected to grow significantly during the forecast period (2024–2034), owing to the launch of upcoming therapies and the increasing prevalence of the disease. With the launch of emerging therapies, many new players are expected to enter the cell and gene therapy in Parkinson's disease market space.
/EIN News/ -- New York, USA, July 22, 2024 (GLOBE NEWSWIRE) -- Cell and Gene Therapy in Parkinson's Disease Market to Register Stunning Growth During the Study Period (2020–2034) | DelveInsight
The cell and gene therapy in Parkinson's disease market size is projected to grow significantly during the forecast period (2024–2034), owing to the launch of upcoming therapies and the increasing prevalence of the disease. With the launch of emerging therapies, many new players are expected to enter the cell and gene therapy in Parkinson's disease market space.
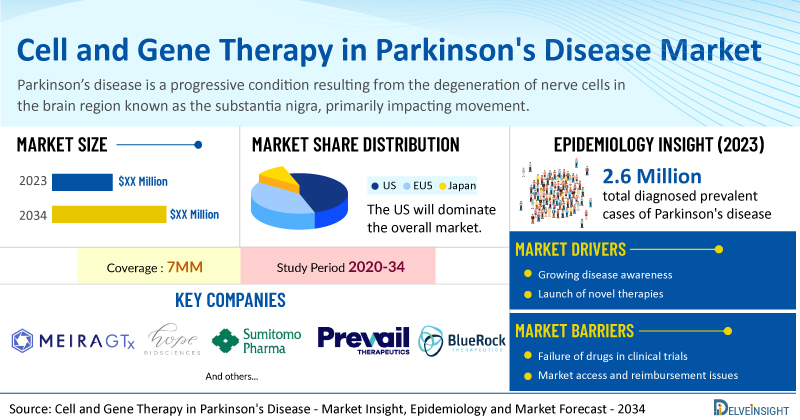
DelveInsight’s Cell and Gene Therapy in Parkinson's Disease Market Insights report includes a comprehensive understanding of current treatment practices, emerging cell and gene therapy in Parkinson's disease, market share of individual therapies, and current and forecasted cell and gene therapy in Parkinson's disease market size from 2020 to 2034, segmented into 7MM [the United States, the EU-4 (Italy, Spain, France, and Germany), the United Kingdom, and Japan].
Key Takeaways from the Cell and Gene Therapy in Parkinson's Disease Market Report
- According to DelveInsight’s analysis, the market size of cell and gene therapy in Parkinson's disease in the 7MM is expected to grow at a significant CAGR by 2034.
- As per DelveInsight analysis, the total diagnosed prevalent cases of Parkinson's disease in the 7MM were found to be 2.6 million cases in 2023, which are expected to increase by 2034 during the study period (2020–2034).
- Prominent companies working in the domain of cell and gene therapy in Parkinson's disease, including MeiraGTx, Hope Biosciences, Sumitomo Pharma, Prevail Therapeutics, BlueRock Therapeutics, Voyager Therapeutics, and others, are actively working on innovative cell and gene therapy in Parkinson's disease. These novel cell and gene therapies in Parkinson's disease are anticipated to enter the cell and gene therapy in Parkinson's disease market in the forecast period and are expected to change the market.
- Some of the key cell and gene therapies in Parkinson's disease treatment include AAV-GAD, HB-adMSCs, CT1-DAP001/DSP-1083, PR001 (LY3884961), Bemdaneprocel (BRT-DA01), VY-AADC, and others.
Discover which therapies are expected to grab the cell and gene therapy in Parkinson's disease market share @ Cell and Gene Therapy in Parkinson's Disease Market Report
Cell and Gene Therapy in Parkinson's Disease Overview
Parkinson’s disease is a progressive condition resulting from the degeneration of nerve cells in the brain region known as the substantia nigra, primarily impacting movement. Motor symptoms of Parkinson’s include tremors, slowness of movement (bradykinesia), stiffness, and postural instability. Non-motor symptoms can encompass cognitive impairment, mood disorders, sleep problems, and autonomic dysfunction. If left unmanaged, these symptoms can significantly reduce a patient's quality of life. Additionally, the progressive nature of the disease imposes a considerable burden on caregivers.
The exact cause of Parkinson’s disease remains unknown, but it is believed to result from a combination of environmental risk factors and genetic predisposition. Men are slightly more prone to the disease than women, and it usually affects individuals over 60, though early-onset cases do occur.
The main pathology involves the degeneration of dopamine-producing neurons in the substantia nigra. Additionally, the buildup of misfolded alpha-synuclein protein in the brain leads to the formation of Lewy bodies, which are characteristic of Parkinson’s disease. Genetic research has identified numerous disease-causing genes and risk factors, confirming the genetic component of Parkinson’s.
Diagnosis is mainly clinical, based on the presence of typical motor and non-motor symptoms. Neuroimaging techniques, such as dopamine transporter (DAT) scans, MRI, PET, and DaTSCAN-SPECT, can aid in supporting the clinical assessment.
Cell and Gene Therapy in Parkinson's Disease Epidemiology Segmentation
The cell and gene therapy in Parkinson's disease epidemiology section provides insights into the historical and current cell and gene therapy in Parkinson's disease patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The cell and gene therapy in Parkinson's disease market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Diagnosed Prevalent Cases of Parkinson's Disease
- Gender-specific Diagnosed Prevalent Cases of Parkinson's Disease
- Age-specific Diagnosed Prevalent Cases of Parkinson's Disease
- Stage-specific Diagnosed Prevalent Cases of Parkinson's Disease
Download the report to understand which factors are driving cell and gene therapy in Parkinson's disease epidemiology trends @ Cell and Gene Therapy in Parkinson's Disease Epidemiological Insights
Cell and Gene Therapy in Parkinson's Disease Treatment Market
Current treatments for Parkinson's disease alleviate symptoms by using medication to replenish lost dopamine, along with disease management and lifestyle interventions like exercise and complementary therapies. Various companies are exploring the promise of experimental gene therapies aimed at regenerating or substituting dopamine, or rescuing dying cells. Additionally, cell-based approaches targeting the replacement or protection of dopaminergic neurons are being considered as potential treatments for Parkinson’s disease. Numerous preclinical studies with animal models have indicated that mesenchymal stem cells (MSCs) could offer potential benefits for this condition.
Despite the introduction of levodopa, few significant advancements have been made in Parkinson’s disease treatment, and several unmet needs persist. The current market lacks curative and disease-modifying therapies, relying solely on symptomatic treatments through a multidisciplinary approach. Even with decades of progress in medications and neurosurgical methods, there remains a need for better symptomatic motor control. Improving control over tremors, gait and balance, posture, dexterity, and communication skills remains a significant challenge in the treatment of Parkinson’s disease movement disorder. Additionally, addressing psychosis in Parkinson’s disease patients is another unmet need.
Learn more about the FDA-approved cell and gene therapies in Parkinson's disease @ Cell and Gene Therapy in Parkinson's Disease Treatment
Emerging Cell and Gene Therapies in Parkinson's Disease and Companies
The cell and gene therapy in Parkinson’s disease market has a promising outlook. The current emerging pipeline is robust; with various therapies being developed, some of which include MeiraGTx’s AAV-GAD, Hope Biosciences’ HB-adMSCs, Sumitomo Pharma’s CT1-DAP001/DSP-1083, Prevail Therapeutics’ (Eli Lilly) PR001 (LY3884961), BlueRock Therapeutics’ Bemdaneprocel (BRT-DA01), Voyager Therapeutics’ VY-AADC, and others.
PR001 is an investigational gene therapy designed to be a potentially disease-modifying, single-dose treatment for Parkinson's disease patients with GBA1 mutations (PD-GBA) and neuronopathic Gaucher disease (nGD). It employs an AAV9 vector and is administered via intra-cisterna magna injection. The GBA1 gene encodes the lysosomal enzyme beta-glucocerebrosidase (GCase), essential for breaking down and recycling glycolipids, which tend to accumulate with age. Individuals with PD-GBA have mutations in at least one copy of the GBA1 gene. The PROPEL Phase I/II clinical trial is currently recruiting participants to assess the safety of intracisternal LY3884961 administration in patients with moderate to severe Parkinson's disease with at least one pathogenic GBA1 mutation. The FDA has granted fast-track designation to PR001 for treating Parkinson’s disease with GBA1 mutations.
AAV-GAD is an experimental gene therapy designed to deliver the glutamic acid decarboxylase (GAD) gene to the subthalamic nucleus to enhance the production of GABA, the brain's main inhibitory neurotransmitter. Since GAD is crucial for GABA synthesis, it's believed that boosting GAD expression in the subthalamic nucleus via gene therapy can normalize motor circuits and improve symptoms in Parkinson’s disease patients without impacting other brain areas associated with current treatment complications. MeiraGTx acquired AAV-GAD from Vector Neurosciences. The drug is undergoing a Phase I/II trial to assess the safety and tolerability of adeno-associated virus-mediated GAD gene delivery in Parkinson's patients. Additionally, discussions with global regulatory bodies are in progress for a pivotal study potentially starting in 2024. Notably, AAV-GAD is the first gene therapy for Parkinson's Disease with an objective imaging biomarker that correlates with clinical improvement.
The anticipated launch of these emerging therapies are poised to transform the cell and gene therapy in Parkinson's disease market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the cell and gene therapy in Parkinson's disease market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about cell and gene therapy in Parkinson's disease clinical trials, visit @ Cell and Gene Therapy in Parkinson's Disease Treatment Drugs
Cell and Gene Therapy in Parkinson's Disease Market Dynamics
The cell and gene therapy in Parkinson's disease market dynamics are anticipated to change in the coming years.
Furthermore, many potential therapies are being investigated for the treatment of Parkinson's disease, and it is safe to predict that the treatment space will significantly impact cell and gene therapy in Parkinson's disease market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate are expected to drive the growth of cell and gene therapy in Parkinson's disease market in the 7MM.
However, several factors may impede the growth of cell and gene therapy in Parkinson's disease market.
Moreover, cell and gene therapy in Parkinson's disease treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the cell and gene therapy in Parkinson's disease market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impactcell and gene therapy in Parkinson's disease market growth.
| Cell and Gene Therapy in Parkinson's Disease Report Metrics | Details |
| Study Period | 2020–2034 |
| Cell and Gene Therapy in Parkinson's Disease Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Cell and Gene Therapy in Parkinson's Disease Companies | MeiraGTx, Hope Biosciences, Sumitomo Pharma, Prevail Therapeutics, BlueRock Therapeutics, Voyager Therapeutics, and others |
| Key Cell and Gene Therapies in Parkinson's Disease | AAV-GAD, HB-adMSCs, CT1-DAP001/DSP-1083, PR001 (LY3884961), Bemdaneprocel (BRT-DA01), VY-AADC, and others |
Scope of the Cell and Gene Therapy in Parkinson's Disease Market Report
- Cell and Gene Therapy in Parkinson's Disease Therapeutic Assessment: Cell and Gene Therapy in Parkinson's Disease current marketed and emerging therapies
- Cell and Gene Therapy in Parkinson's Disease Market Dynamics: Conjoint Analysis of Emerging Cell and Gene Therapy in Parkinson's Disease Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Cell and Gene Therapy in Parkinson's Disease Market Access and Reimbursement
Discover more about cell and gene therapies in Parkinson's disease in development @ Cell and Gene Therapy in Parkinson's Disease Clinical Trials
Table of Contents
| 1. | Cell and Gene Therapy in Parkinson's Disease Market Key Insights |
| 2. | Cell and Gene Therapy in Parkinson's Disease Market Report Introduction |
| 3. | Cell and Gene Therapy in Parkinson's Disease Market Overview at a Glance |
| 4. | Cell and Gene Therapy in Parkinson's Disease Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Cell and Gene Therapy in Parkinson's Disease Treatment and Management |
| 7. | Cell and Gene Therapy in Parkinson's Disease Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Cell and Gene Therapy in Parkinson's Disease Marketed Drugs |
| 10. | Cell and Gene Therapy in Parkinson's Disease Emerging Drugs |
| 11. | Seven Major Cell and Gene Therapy in Parkinson's Disease Market Analysis |
| 12. | Cell and Gene Therapy in Parkinson's Disease Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
| Parkinson's Disease Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Parkinson's Disease Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Parkinson's Disease Epidemiology Segmentation |
|
| Key Parkinson's Disease Companies | Cerevel Therapeutics, Pfizer, Pharma Two B, AbbVie, Biogen, Denali Therapeutics, Annovis Bio, Amneal Pharmaceuticals, BioVie, Cerevance, Clene Nanomedicine, Intra-Cellular Therapies, Hoffmann-La Roche, Prothena Corporation, and others |
| Key Parkinson's Disease Therapies | SPN-830 (apomorphine infusion device), Tavapadon, ABBV-951 (foscarbidopa/foslevodopa), IPX203 (carbidopa/levodopa extended-release), P2B001 (extended-release pramipexole and rasagiline), ND0612 (levodopa/carbidopa), Buntanetap (ANVS401/posiphen), and others |
Parkinson's Disease Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Parkinson's disease companies, including Cerevel Therapeutics, Inhibikase Therapeutics, Neuraly, Peptron, Biogen, Roche, Brain Neurotherapy Bio, Inc., Modag, Annovis Bio Inc., BioVie Inc., United Neuroscience Ltd., Luye Pharma Group, AbbVie, UCB Biopharma SRL, InnoMedica Schweiz AG, Integrative Research Laboratories AB, H. Lundbeck A/S, Shanghai WD Pharmaceutical Co., Ltd., Cerevance Beta, Inc., Nobilis Therapeutics Inc., BlueRock Therapeutics, Taiwan Mitochondrion Applied Technology Co., Ltd., among others.
Parkinson's Disease Epidemiology Forecast
Parkinson's Disease Epidemiology Forecast – 2032 report provides historical as well as forecasted epidemiology segmented by total diagnosed prevalent cases of Parkinson’s disease, gender-specific diagnosed prevalent cases of Parkinson’s disease, age-specific diagnosed prevalent cases of Parkinson’s disease, and stage-specific diagnosed prevalent cases of Parkinson’s disease in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2019 to 2032.
Psychosis in Parkinson’s and Alzheimer’s Disease Market
Psychosis in Parkinson’s and Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key psychosis in Parkinson’s and Alzheimer’s disease companies, including Sunovion Pharmaceuticals, Karuna Therapeutics, Vanda Pharmaceuticals, Suven Life Sciences, Enterin, Intra-Cellular Therapies, Merck Sharp & Dohme, among others.
Parkinson’s Disease-Related Dementia Market
Parkinson's Disease-Related Dementia Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Parkinson’s disease-related dementia companies, including AbbVie, UCB Biopharma SRL, InnoMedica Schweiz AG, Integrative Research Laboratories AB, H. Lundbeck A/S, Shanghai WD Pharmaceutical Co., Ltd., Cerevance Beta, Inc., among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

